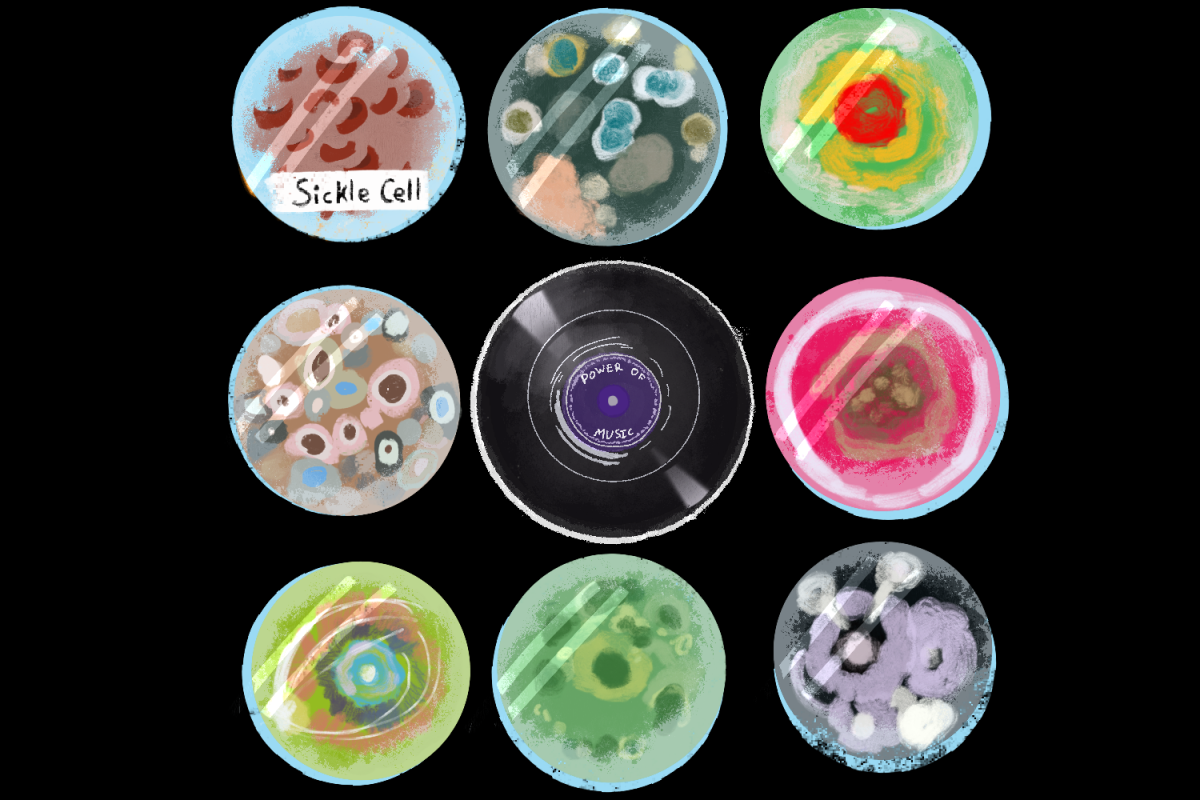
In the past four years, 15 companies that offered direct-to-consumer personal genome tests have stopped sales, and only two other companies continue to provide results on genetic markers for health risks. Just one of these, 23andme, still uses an at-home collection method. Until this past November, the company marketed genomic tests as a method of learning about both ancestry and potential risk of health problems. These risks can be determined by known genetic indicators of conditions such as Alzheimer’s, some cancers and cardiovascular disease. The Food and Drug Administration ordered 23andme to discontinue marketing its personal genome service, with a letter citing concerns over users’ interpretation of the health results and a lack of data to support the tests’ accuracy.
The FDA alleges that a consumer receiving a false positive BRCA-related risk assessment — an indicator for breast/ovarian cancer — might then unnecessarily undergo “prophylactic surgery, chemoprevention, intensive screening or other morbidity-inducing actions.” Yet, none of these are available without a doctor’s approval. The most extreme action that could result is a visit to the doctor that might lead to treatment after testing. Any initiative that results in the discussion of preventative measures with a doctor should be encouraged, not restricted.
The FDA regulates many other at-home biological tests, including the OraQuick In-Home HIV Test. Approved in 2012, this test uses saliva to provide an HIV diagnosis within 40 minutes. Results are then displayed on a stick similar to a pregnancy test. Receiving news of a positive HIV diagnosis is far more traumatic than testing positive for a potential disease risk factor. The FDA has determined that consumers are smart enough to know what to do next concerning their HIV status, so why are they reluctant to do the same for genetic markers? Considering that several studies have indicated there are no changes in users’ psychological health after receiving genomic testing results, the FDA should not prevent consumers from having access to these tests.
The FDA is entirely justified in its regulatory concerns with private genomic testing. Warning labels do need to clearly state that positive or negative results are only partial indicators for some risk factors and that the tests may not be completely accurate. 23andme did not entirely cooperate with the FDA’s request for data and warning label changes and is by no means the perfect model of a genomic testing company. However, the FDA has given ridiculous reasons for completely halting these tests, making it more difficult for companies to provide informative home tests in the future. The government should encourage individuals to take charge of their own health before symptoms develop, not provide excuses to limit access to valid testing.
A version of this article appeared in the Tuesday, Feb. 25 print edition. Tess Woosley is a contributing columnist. Email her at [email protected].